When Pool Chemicals Go Wrong: A High-Rise Hazmat Response with Chlorine Gas
I sat down with Mike Monaco for what we call a “hot wash” episode – where we unpack real incidents shared anonymously by first responders. This time, it was a story from a Midwestern firefighter about a chlorine gas incident inside a high-rise building. Nothing earth-shattering on paper – just a pool chemical mishap – but in practice, it turned into a complex, high-stakes response. And it holds lessons for every hazmat technician out there.
The Call: Chlorine Smell on the Fourth Floor
The ticket came in as a “chlorine smell from the pool.” Not an uncommon report in our world, but always one worth checking out. Turns out, the incident happened in a high-rise building with a pool on the fifth floor and a mechanical room below it on the fourth. That’s where the mix-up happened.
In that room, roughly five gallons of muriatic acid (a weaker form of hydrochloric acid) had been dumped into a 55-gallon drum containing about ten gallons of sodium hypochlorite – your basic liquid pool bleach. Together, those two created a redox (oxidation-reduction) reaction that produced chlorine gas, the same greenish-yellow toxic gas you’d find in an industrial one-ton cylinder – just on a much smaller, but still dangerous, scale.
Not All Chlorine Is Created Equal
One of the first takeaways here is how often people use the word “chlorine” without precision. Pool workers will say “chlorine” when they mean bleach, or sodium hypochlorite. This kind of confusion can lead to dangerous misunderstandings. So one of the immediate lessons from the call: Always confirm the actual chemicals involved, don’t just go by what someone calls them.
Inside the Room: What the Entry Team Saw
The team entered the fourth floor with full bunker gear and SCBA. Smartly, they brought a Draeger XAM5600 chlorine meter and a thermal imaging camera (TIC). These were key.
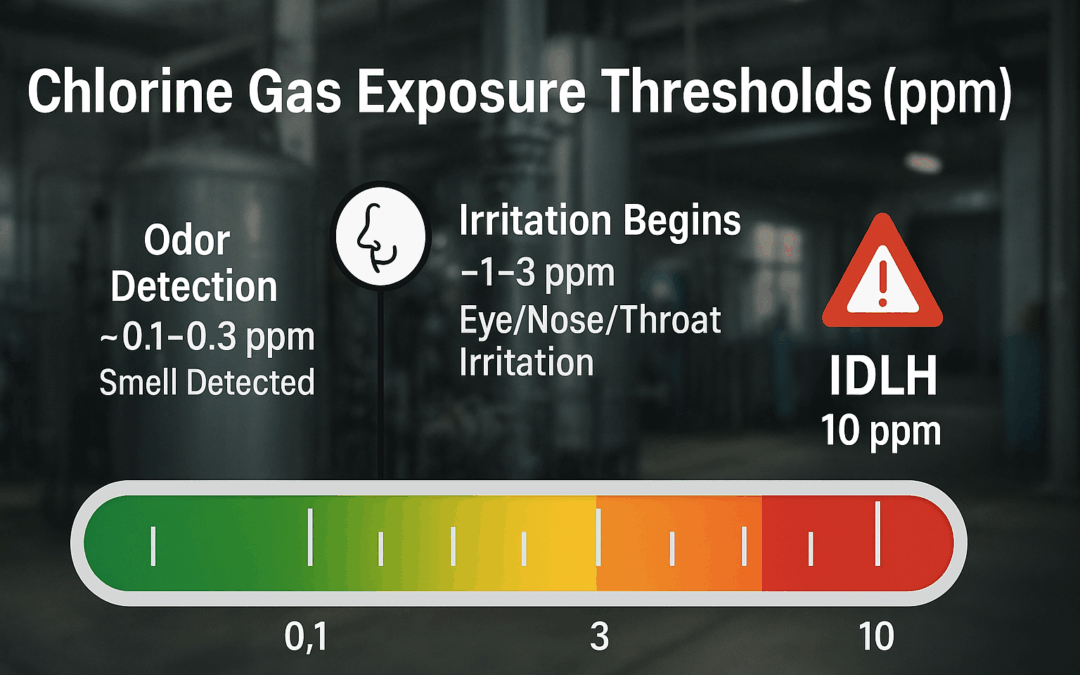
As soon as they got into the hallway outside the mechanical room, their meters started reading 30 parts per million (ppm) of chlorine. Inside the room? It peaked the meter.
They also used the TIC on the drum and picked up a temperature around 99°F, which was well above ambient temperature. That was the first solid confirmation that a chemical reaction was still actively occurring.
Here’s the thing: when you’re using a TIC during a chemical event, the trend in temperature matters more than the number. A steady temp means the reaction is stable. Rising temp means it’s still ramping up. Dropping temp? The reaction is winding down. But even a steady temp can lull you into a false sense of security – it can suddenly spike if you’re not watching.
The Real Problem: Nowhere to Vent
Here’s where things got tricky. Normally, if a reaction like this happens in a residential setting or an outdoor pool shed, you can vent the gas to the outside. But this was a high-rise building, and the mechanical room had no outside ventilation. To get the drum out, they would’ve had to either take it down the stairwell or the elevator, both of which ran through public areas of the building.
That’s when the team made the call: move the drum up – not down – and dump it in the pool.
The Pool Plan: Controlled Dilution
At first glance, it might sound odd – putting reactive chemicals into a pool – but this was smart. The pool was 32,000 gallons, and chemically speaking, dilution slows everything down. You’re separating the acid and bleach molecules so far apart in all that water that the reaction becomes negligible.
Of course, it wasn’t a risk-free operation. As they were hauling the drum up the stairs (since it didn’t fit the drum cart), one firefighter had their mask knocked loose and got a small exposure. That near-miss was a big takeaway for the crew – even well-secured gear can fail when you’re carrying heavy, awkward loads on stairs.
Ventilation & Monitoring: Moving the Gas Out Safely
Once the drum’s contents were in the pool, the reaction continued – but at a far lower intensity. The pool area briefly peaked at 50 ppm of chlorine, but thanks to a planned ventilation route (through a recreation area and out a balcony), the team was able to push the gas out of the building safely.
They used positive pressure fans to direct airflow away from occupied areas and out the external doors. It took about three hours to get the chlorine levels in the pool area back down to 0.5 ppm, well below the 1 ppm ceiling limit for worker safety.
What They Didn’t Do: Neutralization
One responder floated the idea of neutralizing the acid with a base, but they ultimately decided against it. Smart move. Adding another chemical to an already unstable mix is like flipping a coin with a lit match in your hand. Instead, they stuck to dilution and physical removal of the hazard, which kept them in control of the situation.
Communications Hiccup: Radios Fail, Cell Phones Save
There was a breakdown in communication between the command post and the entry teams due to thick concrete walls and high-rise layout. What saved the day? Good old cell phones. When radios wouldn’t work, they used voice calls and texts to stay in contact. It’s a reminder that sometimes Plan B is already in your pocket.
Key Lessons from This Incident
There’s no shortage of takeaways from this call, but here are a few that stuck with me:
- Thermal imaging is essential for understanding chemical reactions in real time.
- Confirm chemical names and reactions before taking action. Don’t rely on what people think they mixed.
- Have a plan for ventilation, especially in confined structures.
- Dilution isn’t always fancy, but it’s often the smartest path forward.
- Backups matter. Whether it’s comms or PPE, be ready for failures.
- Training for real-world scenarios makes a huge difference.